Evaluating the susceptibility and risk of amphibian species to Batrachochytrium salamandrivorans (Bsal) in North America.
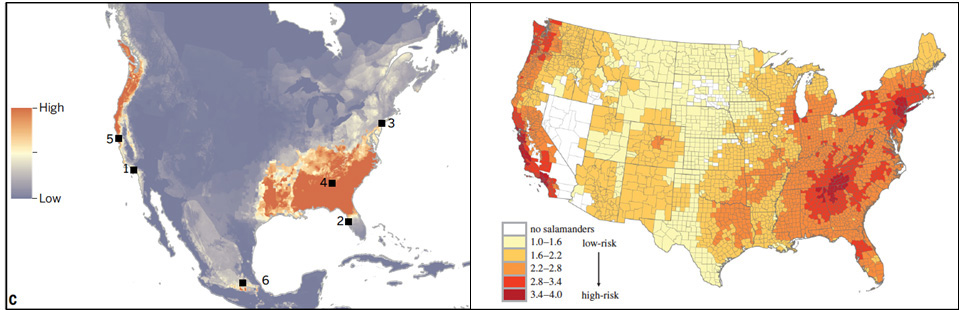
Risk models based on salamander species richness and environmental suitability of Bsal zoospores suggest likely regions of emergence are the southeastern and northwestern United States and southern Mexico. There is urgent need to estimate the susceptibility of salamander species in these regions to Bsal for more robust science-based risk assessments, which can provide guidance to natural resource agencies in developing disease intervention strategies.
Progress: As of September 2018, we have completed Bsal susceptibility trials on 30 amphibian species (24 salamander and six frog species from nine families); three of those species are from Mexico. Future efforts include testing 18 additional species (ten eastern USA, eight western USA) in collaboration with Washington State University. We also are collaborating with University of Georgia to test the susceptibility of salamander species from Costa Rica.
Collaborators:
- Priya Nanjappa
- Reid Harris
- Lori Williams
- Pandy Upchurch
- Doug Woodhams
- Molly Bletz
- Louise Rollins-Smith
- Jonah Piovia-Scott
- Sonia Hernandez
- Henry Adams
- Gabriella Parra Olea
- Tim Herman
- Jessi Krebs
- Diane Barber
Funding:
- BAND Foundation
- Association of Fish and Wildlife Agencies
- North Carolina Wildlife Resources Agencies
- Tennessee Wildlife Resources Agency
- US Fish and Wildlife Service
- Liquid Spark
Susceptibility of North American newts to Batrachochytrium salamandrivorans among species, populations, and age classes.
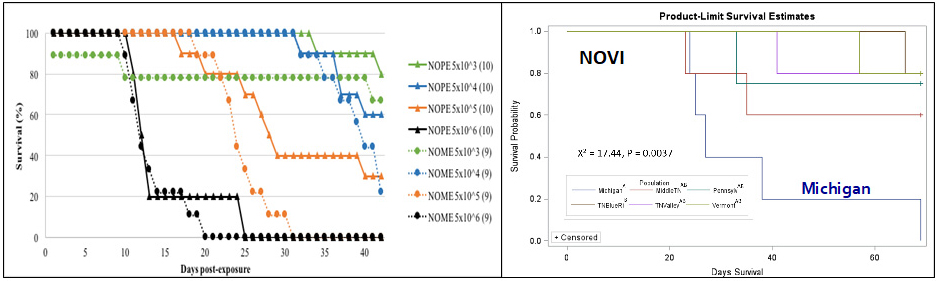
Progress: The recently discovered fungal pathogen Batrachochytrium salamandrivorans (Bsal) has caused population declines of wild fire salamanders (Salamandra salamandra) in Europe, and preliminary controlled experiments suggest that Bsal is highly pathogenic to species in the family Salamandridae (i.e., newts). Our goal was to robustly estimate the susceptibility of four North American newt species (Notophthalmus viridescens, N. perstriatus, N. meridionalis, and Taricha granulosa) to Bsal infection and chytridiomycosis. For N. viridescens, we were able to test the susceptibility of six populations from four US states. Animals were exposed to one of four zoospore doses (5×103-6) at 15o C for 24 hours, and their condition and fate were monitored for at least six weeks.
In general, the greatest mortality, infection prevalence, and Bsal loads were in the following order: N. meridionalis (greatest), N. perstriatus, Taricha granulosa, and N. viridescens (least). In addition, susceptibility differed among N. viridescens populations, with the greatest occurrence of Bsal chytridiomycosis occurring in the Michigan population and least in the Blue Ridge physiographic region, Tennessee (100 percent vs. 20 percent mortality at the highest spore dose, respectively). Interestingly, mucosome inactivation of Bsal zoospores by Michigan newts was lower than Tennessee newts. N. viridescens efts also had higher susceptibility than adult N. viridescens populations. Our results indicate that newt species could play a significant role in the epidemiology of Bsal if it emerges in North America. Moreover, Bsal represents a significant conservation risk to N. meridionalis and N. perstriatus, which are species of greatest conservation need in the southern United States.
Collaborators:
- Doug Woodhams
- Molly Bletz
- Louise Rollins-Smith
Funding:
- University of Tennessee Institute of Agriculture
- University of Massachusetts-Boston
- Vanderbilt University
Transmission pathways and immunological factors driving invasion potential of the recently discovered pathogen, Batrachochytrium salamandrivorans
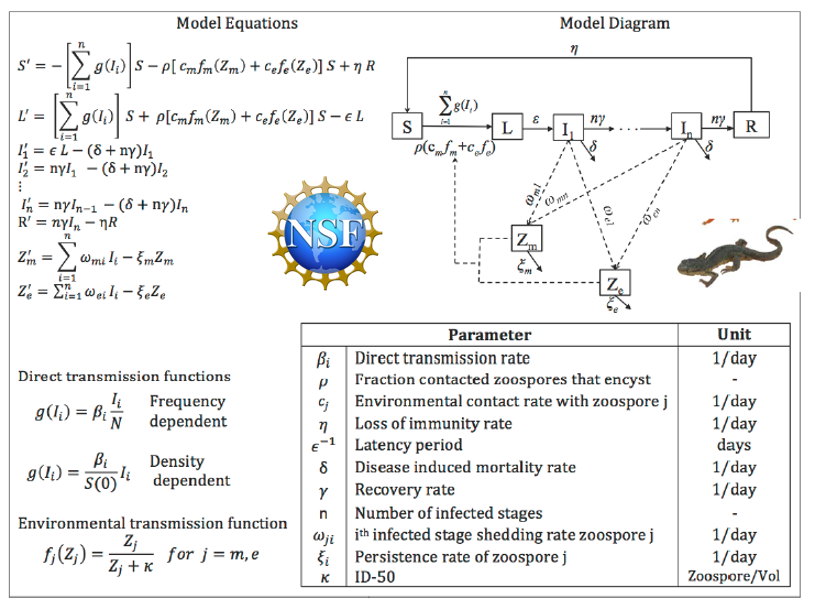
The current knowledge of Bsal epidemiology is restricted mostly to European fire salamanders, which have different life history strategies than most North American salamander species. This project will parameterize Bsal epidemiological models using controlled experiments with adult and juvenile eastern newts (Notophthalmus viridescens).
This study will be the first to examine the effects of Bsal on a common salamander species with complex life histories and immune responses. This research will expand on the S. salamandra model system by:
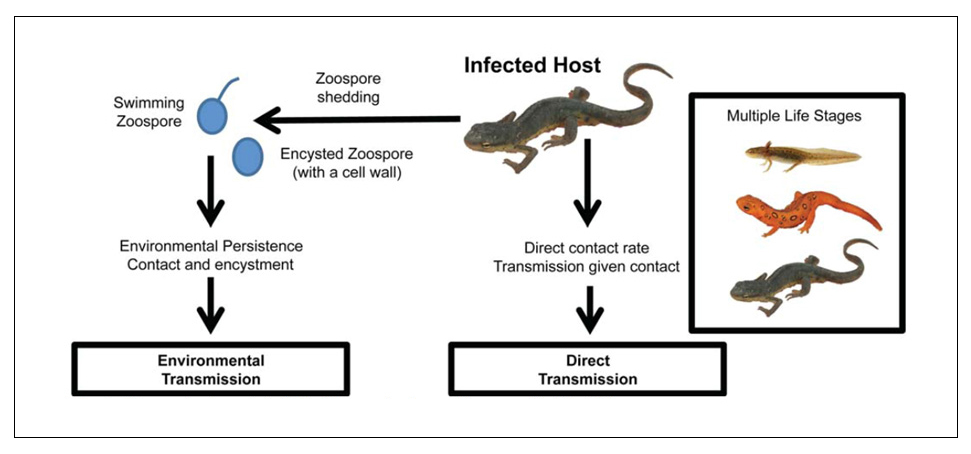
- Including transmission pathways in both the aquatic and terrestrial environments for a range of ambient temperatures,
- Considering two unique forms of infectious Bsal zoospores,
- Identifying the role of zoospore persistence in water and soil and factors that affect it,
- Explicitly modeling zoospore shedding and between-host transmission as disease progresses, and
- Incorporating a recovery stage as an infection outcome.
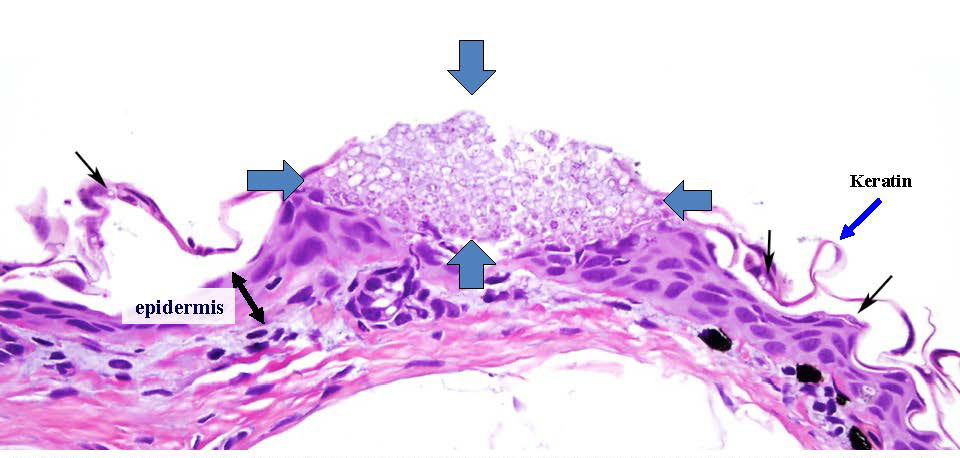
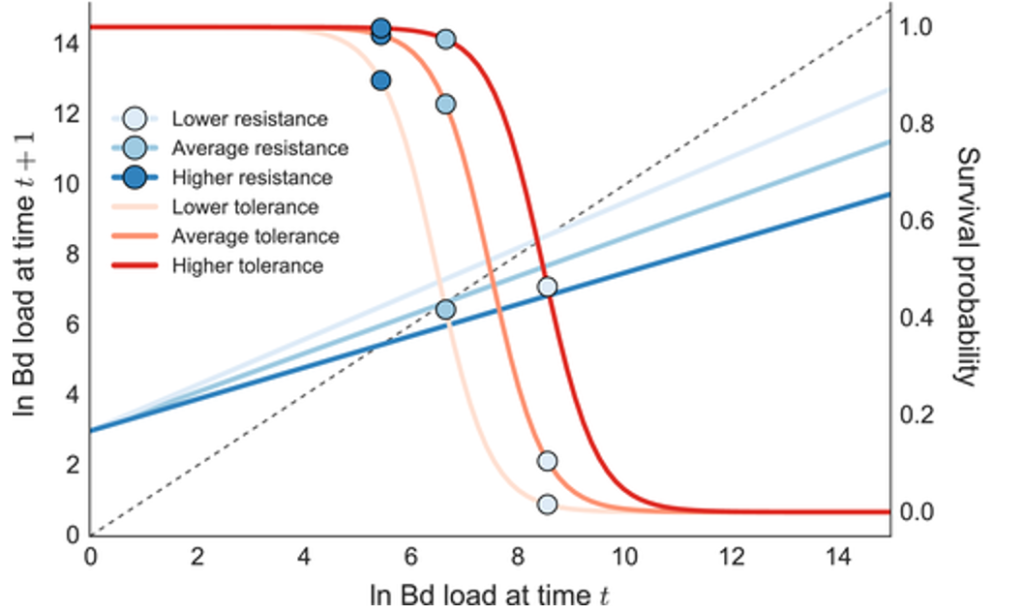
The study will evaluate immune defenses and anatomical pathology as disease progresses, and it will determine if host resistance or tolerance to Bsal infection is possible. Clinical pathology will be used to identify mechanisms of Bsal pathogenesis, which are currently unknown. This research will significantly advance the foundational knowledge of Bsal epidemiology, immunology, and pathology, and will provide the basis for science-derived disease intervention strategies that are generalizable across similar at-risk species.
Key Questions:
- What are the most important transmission pathways that facilitate Bsal invasion, diseased-induced population declines, and pathogen persistence?
- What role does life stage play in emergence and persistence of Bsal?
- How do mitigation strategies and changes in environmental conditions affect transmission and disease outcomes?
- What conditions result in host resistance or tolerance?
- What are the mechanisms of pathogenesis?
Collaborators:
- Doug Woodhams
- Louise Rollins-Smith
- Angie Peace
- Cherie Briggs
- Allan Pessier
Funding:

US National Science Foundation (Division of Environmental Biology – Ecology of Infectious Diseases Program), Grant #1814520
Research Objectives
Broader Impacts
This study will inform the European Food Safety Authority of the European Commission, U.S. Fish and Wildlife Service, and other natural resource management agencies on how to combat biodiversity losses due to Bsal. Research briefings will be provided regularly to the North American Bsal Task Force and the Partners in Amphibian and Reptile Conservation. Our results will be shared at national and international scientific meetings, and updates will be posted on this project website. Our team will train biologists about Bsal via workshops and will engage K-12 STEM schools with instructional activities on amphibians and wildlife diseases. This project will train undergraduate, graduate, and veterinary students, postdoctoral research associates, and veterinary medicine residents. Due to the interdisciplinary nature of this research, trainees will be exposed to: design of factorial experiments, compartmental and integral projection models, analysis of complex microbiomes, gross evaluation of animal health, molecular diagnostics, anatomical and clinical pathology, immunology assays, mass spectrometry, flow cytometry, and data entry and analysis. In addition, individual mentoring plans will be developed for postdocs. Students, postdocs, and senior personnel will travel among partnering institutions for professional development and to strengthen cohesiveness of the study objectives.