Batrachochytrium salamandrivorans (Bsal) is a recently discovered fungal pathogen emerging in Europe. The pathogen likely originated in Asia, where local species harbor infections without signs of clinical disease (Laking et al. 2017) and is hypothesized to have been introduced to Europe via unregulated international trade. The pathogen has caused widespread die-offs of fire salamanders (Salamandra salamandra; Martel et al. 2013, Stegen et al. 2017), and has been detected in wild populations of alpine newts (Ichthyosaura alpestris) and smooth newts (Lissotriton vulgaris) in Belgium, Germany, and the Netherlands (Spitzen-van der Sluijs et al. 2016), as well as in captive amphibians in Germany (Sabino-Pinto et al. 2015) and the UK (Cunningham et al. 2015).
Experimental challenges show that one or more species in Salamandridae, Plethodontidae, and Sirenidae are suitable hosts for the pathogen, with the greatest disease incidence in Salamandridae species (Martel et al. 2014). Unlike the well-known chytrid fungus, Batrachochytrium dendrobatidis (Bd), Bsal does not appear to be pathogenic to anurans (frogs and toads), although they can become infected and hence may serve as important reservoir species, and facilitate persistence of the pathogen in an amphibian community (Stegen et al. 2017). The zoospores also have strong adhesion properties and can stick to the feet of waterbirds, possibly facilitating long-distance translocation of the pathogen (Stegen et al. 2017).
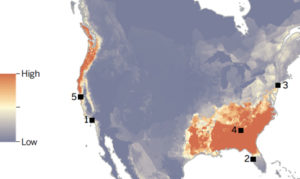
A recent risk analysis suggests that amphibian communities in the southeastern United States may be at greatest risk because environmental conditions are suitable for Bsal, and amphibian species richness is high (Yap et al. 2015; Figure 1). However, the predictions of these models are limited by a lack of information on the host range in North America, and the range of environmental suitability for Bsal may be wider than predicted by laboratory studies (e.g., Laking et al. 2017). Thus, there is urgent need to understand susceptibility of native species to robustly estimate risk in the event that Bsal emerges in North America.
To date, Bsal has not been detected in amphibian populations in North America. However, North America is a global hotspot for salamander biodiversity, accounting for 50 percent of species worldwide (Yap et al. 2015), thus there is substantial concern about the possibility of Bsal’s introduction into the region (Burke et al. 2015; Gray et al. 2015).
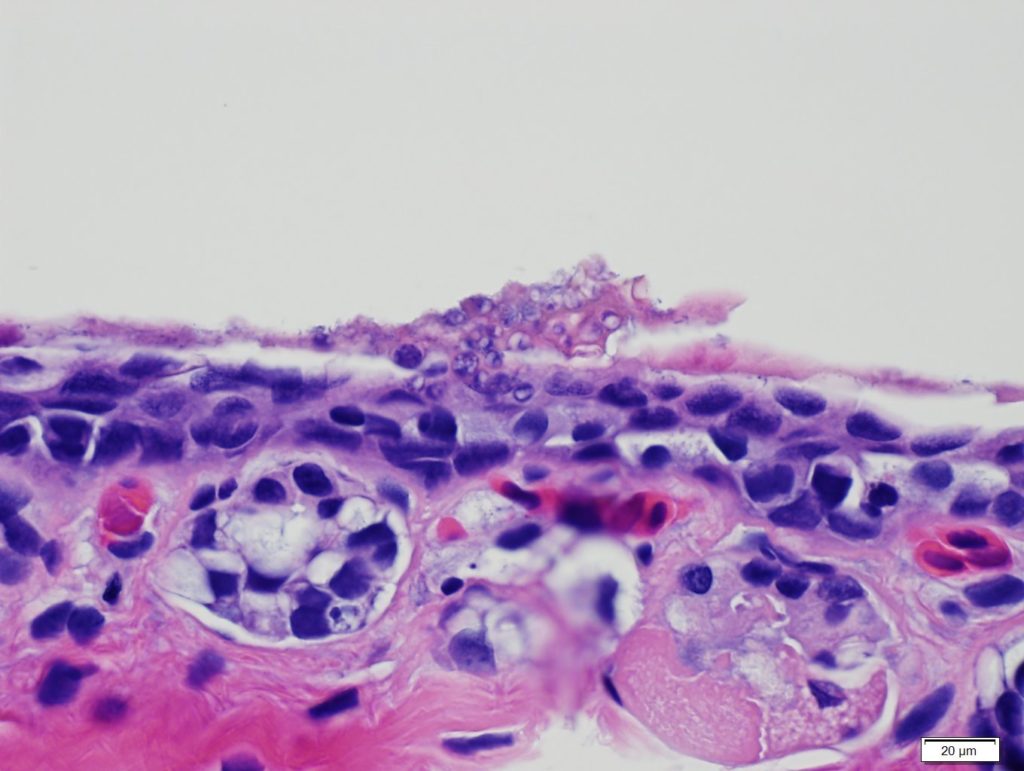
Bsal reproduces asexually and spreads via zoospores, which colonize a host and encyst in the cells of the epidermis. The encysted zoospore then germinates and develops into a thallus, which can release new zoospores to the environment via a discharge tube (Van Rooij et al. 2015). Bsal infection can be characterized by skin lesions, which are associated with large numbers of encysted thalli that spread across the epidermis. Preliminary data suggest that lesions from Bsal chytridiomycosis may present differently between host species, with deep localized lesions in some species (e.g., Salamandra salamandra; Martel et al. 2014) and diffuse infection and superficial lesions in others (e.g., Eurycea wilderae; D. L. Miller, unpublished data).
Other commonly observed signs of disease include inappetence, skin sloughing, and erythema. Because large portions of the epidermis can be destroyed by Bsal, osmoregulation is likely disrupted, which can impair various physiological functions in amphibians and cause death. Diagnosis of Bsal chytridiomycosis is typically achieved by a combination of two qualifiers: detection and quantification of Bsal DNA on either skin swabs or excised skin via qPCR (Blooi et al. 2013) and observations of disease signs via histopathology (Miller et al. 2015).
Despite our understanding of the clinical signs of chytridiomycosis, we know little about immune responses to Bsal infection. The primary defensive mechanism against Bd is mucosal skin secretion, as both a physical and immunological barrier to infection. The amphibian mucosome is composed of several factors that defend against zoospore encystation, including antimicrobial peptides, lysozymes, and symbiotic microbial communities who themselves produce antifungal metabolites (Van Rooij et al. 2015).
Indeed, skin mucosome can reduce the number of viable Bd zoospores in vitro, which, combined with the physical barrier the mucus layer represents, may decrease zoospore colonization of the skin. Thus, the first line of defense against Bsal infection may likely be the skin mucosome.
Recent gene expression data from Farrer et al. (2017) suggest the adaptive immune response to Bsal infection is nonexistent in highly susceptible species, which supports that the pathogen is a novel and recent introduction to northern Europe.

Given the high pathogenicity of Bsal to some salamander species, as well as its presence in international trade (Cunningham et al. 2015), Bsal is a significant threat to salamander biodiversity worldwide. Disease prevalence is variable among species, but there are a number of species that are suitable carrier hosts for the pathogen (including anuran species; Stegen et al. 2017, M. J. Gray, unpublished data) and can spread the pathogen to more susceptible species.
Little is known about the susceptibility of North American species to Bsal, and thus further preemptive action, such as susceptibility trials, Bsal transmission and persistence experiments, and environmental monitoring, is needed to robustly accurately predict risk and develop effective mitigation strategies in the event of Bsal’s emergence in North America.
- Blooi M., Pasmans F., Longcore J. E., Spitzen-van der Sluijy A., Vercammen F., Martel A. (2013). Duplex realtime PCR for rapid simultaneous detection of Batrachochytrium dendrobatidis and Batrachochytrium salamandrivorans in amphibian samples. Journal of Clinical Microbiology 51:4173–4177.
- Burke, K. L. (2015). New disease emerges as threat to salamanders. American Scientist 103:6-7.
- Cunningham A. A., Beckman K., Perkins M., Fitzpatrick L., Cromie R., Redbond J., et al. (2015). Emerging disease in UK amphibians. Veterinary Record 176:468.
- Gray M. J., Lewis J.P., Nanjappa P., Klocke B., Pasmans F., Martel A., Stephen C., Olea G. P., Smith S.A., Sacerdote-Velat A., Christman M. R. (2015). Batrachochytrium salamandrivorans: The North American response and a call for action. PLoS Pathogens 11(12):e1005251.
- Laking A.E., Ngo H.N., Pasmans F., Martel A., Nguyen T.T. (2017). Batrachochytrium salamandrivorans is the predominant chytrid fungus in Vietnamese salamanders. Scientific Reports 7:44443.
- Martel A., Blooi M., Adriaensen C., Van Rooij P., Beukema W., Fisher M.C., et al. (2014). Recent introduction of a chytrid fungus endangers Western Palearctic salamanders. Science 346:630-631.
- Martel A, Spitzen-van der Sluijs A., Blooi M., Bert W., Ducatelle R., Fisher M.C., et al. (2013). Batrachochytrium salamandrivorans sp nov causes lethal chytridiomycosis in amphibians. Proceedings of the National Academy of Sciences of the United States of America 110:15325-15329.
- Miller D. L., Pessier A. P., Hick P., Whittington R. J. (2015). Comparative pathology of ranaviruses and diagnostic techniques. In: Gray M. J., Chinchar VG (eds) Ranaviruses: Lethal pathogens of ectothermic vertebrates. Springer, Secaucus, NJ.
- Richgels K. L., Russell R. E., Adams M. J., White C. L., Grant E. H. (2017). Spatial variation in risk and consequence of Batrachochytrium salamandrivorans introduction in the USA. Royal Society open science. 3(2):150616.
- Sabino-Pinto J., Bletz M., Hendrix R., Perl R. G. B., Martel A., Pasmans F., Lötters S., Mutschmann F., Schmeller D., Schmidt B., Veith M., Wagner N., Vences M., Steinfartz S. (2015). First report of Batrachochytrium salamandrivorans in Germany. Amphibia-Reptilia 36(4):411-416.
- Spitzen-van der Sluijs A., Martel A., Asselberghs J., Bales E. K., Beukema W., Bletz M. C., Dalbeck L., Goverse E., Kerres A., Kinet T., Kirst K. (2016). Expanding distribution of lethal amphibian fungus Batrachochytrium salamandrivorans in Europe. Emerging Infectious Diseases 22(7):1286.
- Stegen G., Pasmans F., Schmidt B. R., Rouffaer L. O., Van Praet S., Schaub M., Canessa S., Laudelout A., Kinet T., Adriaensen .C, Haesebrouck F. (2017). Drivers of salamander extirpation mediated by Batrachochytrium salamandrivorans. Nature 544(7650):353-6.
- Van Rooij P., Martel A., Haesebrouck F., Pasmans F. (2015). Amphibian chytridiomycosis: A review with focus on fungus-host interactions. Veterinary Research 46(1):137.
- Yap T. A., Koo M. S., Ambrose R. F., Wake D. B., Vredenburg V. T. (2015). Averting a North American biodiversity crisis: A newly described pathogen poses a major threat to salamanders via trade. Science 349:481-482.